Researchers have developed a calcium sensor for use in magnetic resonance imaging (MRI) that they hope can one day track electrical activity in neurons deep in living brains. The tool potentially allows scientists an unprecedented view of brain activity in specific types of cells across the entire brain of a living organism (ACS Sensors 2021, DOI: 10.1021/acssensors.1c01085).
“Calcium is the ionic language in which electrical activity in neurons gets converted to biological function,” says Arnab Mukherjee, a chemical engineer at the University of California, Santa Barbara, and the study’s principal investigator. Many groups have developed sensors that detect calcium ions, he says, but most rely on light. That limits their use to the very top few millimeters of the brain that visible or infrared light can penetrate into, and even then, only a small area of the brain can be viewed using a microscope.
“Answers to some of the most interesting questions—whether it’s cognition or emotion or addiction—are buried far deeper than what is accessible using light,” says Mukherjee. “And neuronal connections go several centimeters from one end to another, so you really need to look at the whole brain at once.”
The sensor’s main benefit, says Mukherjee, is that because it is a protein and not a small molecule, it can be genetically encoded into any cell type, making it noninvasive and allowing researchers to study brain processes over long periods of time. The new sensor reveals the location of calcium inside cells by taking advantage of the magnetic properties of naturally present manganese ions. Those properties make manganese detectable by MRI, an imaging technique that can visualize the entire brain.
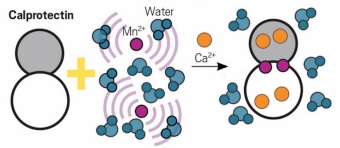
For the sensor, Mukherjee’s team looked to a protein called calprotectin, which helps the immune system by trapping metallic ions—among them, manganese ions—that pathogens require to grow. Calprotectin begins to play this role only when it senses calcium ions. When the protein sequesters manganese ions, it shields them from water. And because MRI works by detecting localized differences in the magnetic field of water, the MRI signal changes based on how much manganese is sequestered, which in turn depends on the concentration of calcium.
In the new study, the researchers packaged DNA encoding calprotectin into a lentivirus and used that virus to deliver the gene into cells in a dish. When the engineered cells began producing calprotectin, they could track the MRI signal based on the calcium concentration in those cells.
The work is “a preliminary proof of concept” that “demonstrates a design or a mechanism by which a change in calcium ion concentration could be changed into an MRI signal,” says Robert Campbell, a chemist at the University of Tokyo who was not involved in the study. Ultimately, though, the aim is to deliver the protein’s DNA into the brain of a living organism “to achieve whole volumetric imaging of a complete animal model brain,” he says
Mukherjee’s team is working on extending the technique to animals and plans to use it to study the neurobiological mechanisms of addiction. Although the sensor will initially be used in animal studies, ones like it may one day be used in humans for certain medical applications, he says—for example, in mapping the brains of people about to undergo brain surgery.
Written by Alla Katsnelson, published in Chemical & Engineering News on September 9, 2021
Image Caption: Calprotectin can be used to sense calcium indirectly. When caprotectin encounters calcium, the protein sequesters paramagnetic manganese ions interacting with surrounding water molecules, altering the water molecules' magnetic fields and changing the MRI signal the water gives off. Credit: Adapted from ACS Sensors



